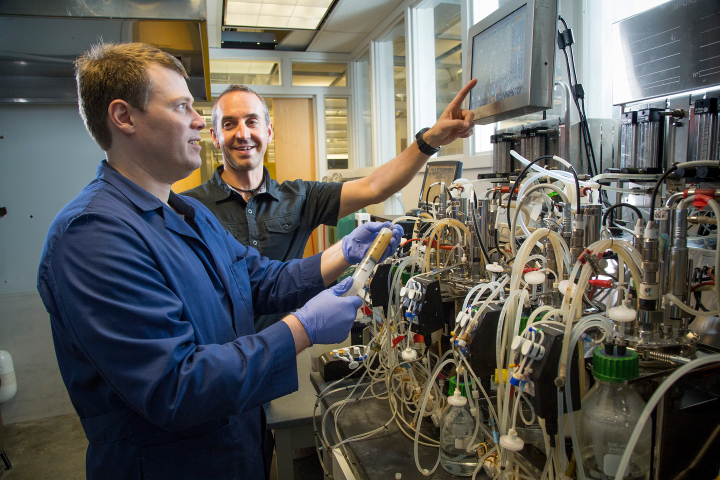
06 February 2020 – DMC Biotechnologies has successfully produced its lead product, L-alanine at pilot scale. Commercial key performance metrics were achieved and performance from the bench scale was also demonstrated at 3m3 scale fermentation and for each of the downstream purification unit operations. The robustness of DMC’s Dynamic Metabolic Control technology to the process environment was also validated.
The predictability of the DMC process has been demonstrated from high throughput screening to commercially relevant scale. Kilogram-sized samples of on-specification product are currently being provided to potential customers, paving the way to commercial launch later this year. No further strain improvement or process engineering is required.
During the execution of the fermentation, a technical failure of an inlet valve in the pilot plant resulted in a material deviation from the protocol. This type of mechanical issue is commonplace in the real world of industrial fermentation. The DMC strain and process were not materially affected by this deviation and the target metrics were still achieved because of the robustness of the technology and the timely response by BPF’s operators. In contrast, most of the current bioprocesses today would have been un-recoverable to this type of deviation.
Raimo van der Linden, Business Development Manager, Bioprocess Pilot Facility (BPF) said, “We recreate real life industrial conditions at BPF and help companies such as DMC validate their technology at pilot scale. The robustness of DMC’s process was thoroughly challenged during this work. The demonstration of robustness to the process environment is truly game-changing for the field of industrial biotechnology.”
Matt Lipscomb, Ph.D., CEO & Co-Founder of DMC said, “Successful pilot scale production is an important step towards full commercialization of L-alanine which will happen later this year. We knew our Dynamic Metabolic Control process was robust. It has been further proven through real-world operating conditions at BPF’s pilot plant.”
See the video press release at https://youtu.be/UvJAyllodRo.
About DMC
DMC is a US biobased specialty chemical company which makes products using microbial fermentation. DMC’s metabolic engineering process simplifies biology to make fermentation more predictable, robust, and efficient. By unleashing the power of biology, DMC is on track to address two of the global challenges of our time: sustainably and affordably feeding a growing world population and reducing greenhouse gas emissions through efficient production of (bio)chemicals. For more information, visit: www.dmcbio.com
DMC media contact:
Kathryn Sheridan
Sustainability Consult
[email protected]